
Chemistry, 27.11.2020 14:00 lillysiege
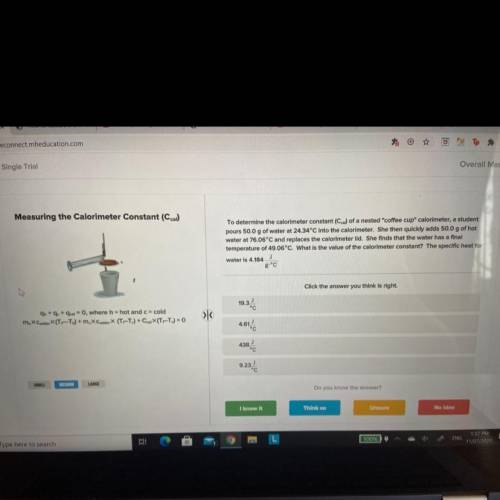
To determine the calorimeter constant (Ccal) of a nested "coffee cup" calorimeter, a student
pours 50.0 g of water at 24.34°C into the calorimeter. She then quickly adds 50.0 g of hot
water at 76.06°C and replaces the calorimeter lid. She finds that the water has a final
temperature of 49.06°C. What is the value of the calorimeter constant? The specific heat for
J
water is 4.184

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
To determine the calorimeter constant (Ccal) of a nested "coffee cup" calorimeter, a student
pours...
Questions


Mathematics, 20.09.2019 00:10

Mathematics, 20.09.2019 00:10


Mathematics, 20.09.2019 00:10




Mathematics, 20.09.2019 00:10

History, 20.09.2019 00:10



Mathematics, 20.09.2019 00:10



English, 20.09.2019 00:10




Mathematics, 20.09.2019 00:10



