
Chemistry, 28.11.2020 05:30 kingalbyss1230
Calculate how many grams of sodium hypochlorite can be theoretically formed when 1.23 mol of sodium hydroxide is combined with 1.36 mol of chlorine.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Calculate how many grams of sodium hypochlorite can be theoretically formed when 1.23 mol of sodium...
Questions

Mathematics, 30.01.2020 14:46


English, 30.01.2020 14:46


Mathematics, 30.01.2020 14:46


Mathematics, 30.01.2020 14:46


History, 30.01.2020 14:46

Mathematics, 30.01.2020 14:46

Social Studies, 30.01.2020 14:46


Mathematics, 30.01.2020 14:46




Mathematics, 30.01.2020 14:46

History, 30.01.2020 14:46


Biology, 30.01.2020 14:46

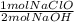 = 0.615 mol NaClO
= 0.615 mol NaClO

