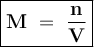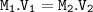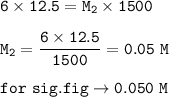Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
12.5 mL of 6.0 M KCl is diluted to make a 1.5 L solution. The molarity of the dilution solution is _...
Questions





Health, 08.01.2021 23:50


History, 08.01.2021 23:50




Mathematics, 08.01.2021 23:50

Mathematics, 08.01.2021 23:50


Mathematics, 08.01.2021 23:50

Health, 08.01.2021 23:50

Mathematics, 08.01.2021 23:50

Mathematics, 08.01.2021 23:50

Medicine, 08.01.2021 23:50

Mathematics, 08.01.2021 23:50

Biology, 08.01.2021 23:50