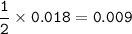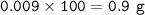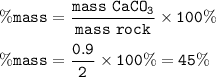Chemistry, 28.11.2020 22:30 nurmukhammada
A piece of rock has a mass of 2.00g. It contains calcium carbonate, but no other
substances. It neutralises exactly 36.0 cm of 0.500 moldmhydrochloric acid.
What is the percentage of calcium carbonate in the 2.00 g piece of rock?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
A piece of rock has a mass of 2.00g. It contains calcium carbonate, but no other
substances. It neu...
Questions

Mathematics, 06.11.2020 01:00



Chemistry, 06.11.2020 01:00

Biology, 06.11.2020 01:00





Mathematics, 06.11.2020 01:00


Mathematics, 06.11.2020 01:00



Mathematics, 06.11.2020 01:00




Biology, 06.11.2020 01:00

History, 06.11.2020 01:00