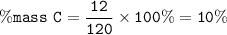Chemistry, 29.11.2020 01:20 axell13965
What percentage of Carbon (C) is present in one molecule of
Dichlorodifluoromethane (CF2Cl2), commonly known as Freon, an anti-freezing agent
used in automobiles.
Please round everything to whole numbers for this question. Use the following
masses in your calculations:
C = 12
F = 19
Cl = 35

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
What percentage of Carbon (C) is present in one molecule of
Dichlorodifluoromethane (CF2Cl2), commo...
Questions

Mathematics, 05.11.2020 22:40





History, 05.11.2020 22:40

Mathematics, 05.11.2020 22:40


Mathematics, 05.11.2020 22:40


Mathematics, 05.11.2020 22:40


Arts, 05.11.2020 22:40

Spanish, 05.11.2020 22:40



Mathematics, 05.11.2020 22:40



History, 05.11.2020 22:40