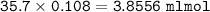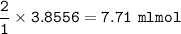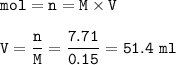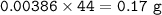Chemistry, 29.11.2020 03:20 elizabethseoane1607
Part A
What volume (in mL ) of a 0.150 M HNO3 solution will completely react with 35.7 mL of a 0.108 M Na2CO3 solution according to the following balanced chemical equation?
In the reaction in Part A, what mass (in grams) of carbon dioxide forms?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
Part A
What volume (in mL ) of a 0.150 M HNO3 solution will completely react with 35.7 mL of a 0.10...
Questions




Mathematics, 04.06.2020 13:07








Mathematics, 04.06.2020 13:07



History, 04.06.2020 13:07


History, 04.06.2020 13:07



Mathematics, 04.06.2020 13:07