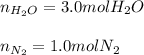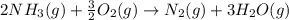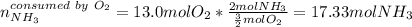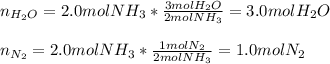Chemistry, 29.11.2020 15:20 westes0376
Ammonia gas(NH3) and oxygen(O2) gas react to form nitrogen gas and water vapor. Suppose you have 2.0 mol of and 13.0 mol of O2 in a reactor. Calculate the largest amount of that could be produced. Round your answer to the nearest .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1


Chemistry, 23.06.2019 06:00
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2

Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
You know the right answer?
Ammonia gas(NH3) and oxygen(O2) gas react to form nitrogen gas and water vapor. Suppose you have 2.0...
Questions


Mathematics, 27.08.2020 04:01

History, 27.08.2020 04:01

Mathematics, 27.08.2020 04:01

History, 27.08.2020 04:01



Mathematics, 27.08.2020 04:01





Arts, 27.08.2020 04:01