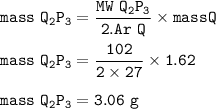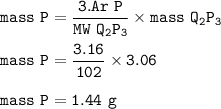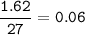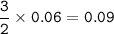Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
What is the mass of element P which reacts with 1 .62g of element Q to form a compound with formula...
Questions

Biology, 27.05.2020 20:57


Health, 27.05.2020 20:57

Mathematics, 27.05.2020 20:57

Biology, 27.05.2020 20:57


English, 27.05.2020 20:57


History, 27.05.2020 20:57




English, 27.05.2020 20:57


Mathematics, 27.05.2020 20:57

Mathematics, 27.05.2020 20:57




History, 27.05.2020 20:57