
Chemistry, 30.11.2020 16:30 Jgarciar6841
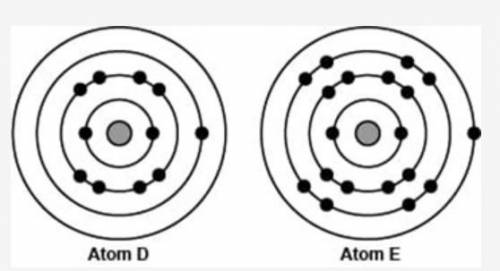
The image compares the arrangement of electrons in two different neutral atoms.
A shaded sphere is shown at the center of four concentric circles. The innermost circle has two black spheres. The middle circle has eight black spheres. The outermost circle has one black sphere. To the right of this figure labeled Atom D is another figure labeled Atom E. Atom E has a shaded sphere at the center of four concentric circles. The innermost circle has two black spheres. The second and the third circles have eight black spheres each. The outermost circle has one black sphere.
Which of the following best explains the position of the two atoms in the periodic table?
A - Both atoms have an estimated Zeff of 1; therefore, Atom D is to the right of Atom E in the same period.
B - Both atoms have Zeff of 1; therefore, Atom D is above Atom E in the same column because of the additional energy level.
C - Atom D has an estimated Zeff of 1 and is therefore to the left of Atom E, which has a Zeff of 9.
D - Atom D has an estimated Zeff of 1 and is therefore below Atom E in the same column, which has a Zeff of 9.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

You know the right answer?
The image compares the arrangement of electrons in two different neutral atoms.
A shaded sphere is...
Questions

History, 28.09.2019 10:50


Physics, 28.09.2019 10:50





Mathematics, 28.09.2019 10:50

English, 28.09.2019 10:50

Mathematics, 28.09.2019 10:50

History, 28.09.2019 10:50



World Languages, 28.09.2019 10:50

History, 28.09.2019 11:00



Mathematics, 28.09.2019 11:00





