Chemistry, 30.11.2020 18:00 queenkimm26
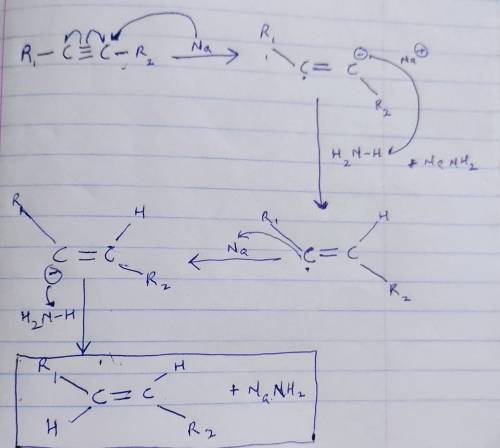
Testbank, Question 098 In the reaction between an alkyne and Na metal in liquid ammonia, the role of Na is as a(n) .
a. catalyst
b. electrophile
c. Brønsted base
d. reducing agent
e. Bronsted acid

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Testbank, Question 098 In the reaction between an alkyne and Na metal in liquid ammonia, the role of...
Questions









Spanish, 23.11.2019 05:31