
Chemistry, 30.11.2020 22:00 shanedietz44ovi6rb
1)26.4 % Carbon
3.3 % Hydrogen
70.3 % Oxygen
Molar Mass: 91.0 g/mol
Empirical Formula:
Molecular Formula:

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1


Chemistry, 23.06.2019 09:00
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
You know the right answer?
1)26.4 % Carbon
3.3 % Hydrogen
70.3 % Oxygen
Molar Mass: 91.0 g/mol
Empirical For...
70.3 % Oxygen
Molar Mass: 91.0 g/mol
Empirical For...
Questions

Computers and Technology, 02.11.2020 22:30






Mathematics, 02.11.2020 22:30


Arts, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30


English, 02.11.2020 22:30


Mathematics, 02.11.2020 22:30



History, 02.11.2020 22:30


English, 02.11.2020 22:30

Mathematics, 02.11.2020 22:30

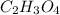 Molecular Formula: (
Molecular Formula: (


