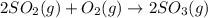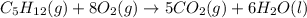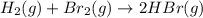Chemistry, 01.12.2020 03:00 AlaskaAirlines
At constant pressure for which of the reactions shown below should Delta H be greater than Delta E°?
1.2 SO2(g) + O2(g) - 2 SO3(g)
II. C5H12(9) + 8 O2(g) - 5 CO2(g) + 6 H20(1)
III. H2(g) + Br2(g) → 2 HBr(9),
IV. N204(9) - 2 NO2(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 23.06.2019 11:30
Distilled water is a completely neutral solution. what is its ph? a. 1 b. 7 c. 14 d. 0
Answers: 2
You know the right answer?
At constant pressure for which of the reactions shown below should Delta H be greater than Delta E°?...
Questions


History, 26.06.2019 00:30

Mathematics, 26.06.2019 00:30


History, 26.06.2019 00:30

Social Studies, 26.06.2019 00:30


Mathematics, 26.06.2019 00:30


Mathematics, 26.06.2019 00:30

History, 26.06.2019 00:30



Biology, 26.06.2019 00:30

Mathematics, 26.06.2019 00:30

Spanish, 26.06.2019 00:30

World Languages, 26.06.2019 00:30

World Languages, 26.06.2019 00:30


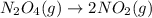 has higher value of
has higher value of  than
than 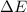
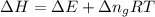
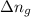 = change in the gaseous moles of the reaction = Moles of product - Moles of reactant
= change in the gaseous moles of the reaction = Moles of product - Moles of reactant