
Chemistry, 01.12.2020 03:20 christianfielding336
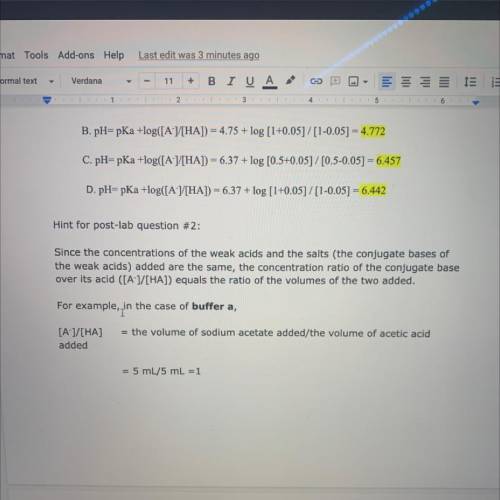
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjugate bases of
the weak acids) added are the same, the concentration ratio of the conjugate base
over its acid ([A-]/[HA]) equals the ratio of the volumes of the two added.
For example, in
in the case of buffer a,
= the volume of sodium acetate added/the volume of acetic acid
[A-]/[HA]
added
= 5 mL/5 mL =1

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
The earth's moon is unusually large. two popular theories of the moon's origin include the "sister world" hypothesis, which states that the moon formed from the same materials as the earth, near enough to the earth that they fell into orbit around each other. a second theory is the "capture" hypothesis, in which the moon formed elsewhere in the solar system, and the earth's gravity pulled it into its orbit. studies of what the moon is made of indicate that some of its materials had to come from the earth or from the same area of the solar system where the earth had formed. at the same time, the moon does not contain much of the material that makes up the earth's core, so the moon could not have formed from the same materials as the earth. how do the two facts above affect the described theories of the moon's origin? a. they show that scientists will never agree on where the moon came from. b. they show that more experiments on moon formation need to be done. c. they show that no theory accounts for the existence of the moon. d. they show that neither theory is complete and entirely correct.
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjug...
Questions







Geography, 22.02.2021 16:10

Mathematics, 22.02.2021 16:10


Mathematics, 22.02.2021 16:10

Mathematics, 22.02.2021 16:10

Health, 22.02.2021 16:10

Social Studies, 22.02.2021 16:10

Social Studies, 22.02.2021 16:10


Computers and Technology, 22.02.2021 16:10


Advanced Placement (AP), 22.02.2021 16:10

English, 22.02.2021 16:10



