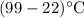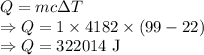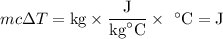Chemistry, 01.12.2020 04:50 kiddshay8232
In order to make 4 cups of tea, 1.00 kg of water is heated from 22.0 oC to 99.0 oC. How much energy is required? 1) One point for how you would set up the problem and correct answer 2) 1 point for explaining any conversions 3) One point for explaining if this is endothermic or exothermic and why

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
In order to make 4 cups of tea, 1.00 kg of water is heated from 22.0 oC to 99.0 oC. How much energy...
Questions

Mathematics, 01.05.2021 02:20




Mathematics, 01.05.2021 02:20



History, 01.05.2021 02:20


Mathematics, 01.05.2021 02:20



Mathematics, 01.05.2021 02:20


History, 01.05.2021 02:20

History, 01.05.2021 02:20



Mathematics, 01.05.2021 02:20

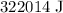
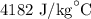
 = Change in temperature =
= Change in temperature =