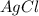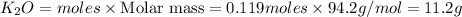Chemistry, 01.12.2020 07:30 ijustneedhelp29
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reagent and what is the theoretical yield of the reaction?
Hint: write the balanced reaction
K - 39.10 g/mol
O - 15.999 g/mol
ANSWER CHOICES:
A.) O2 is limiting, 11.2 g of K2O formed
B.) K is limiting, 14.7 g of K2O formed
C.) K is limiting, 11.2 g of K2O formed
D.) O2 is limiting, 14.7 g of K2O formed
E.) O2 is limiting, 19.2 g of K2O formed

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1

You know the right answer?
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reag...
Questions



Mathematics, 29.05.2021 07:00


Mathematics, 29.05.2021 07:00




Mathematics, 29.05.2021 07:00



Mathematics, 29.05.2021 07:10


Mathematics, 29.05.2021 07:10

Business, 29.05.2021 07:10

Mathematics, 29.05.2021 07:10

Arts, 29.05.2021 07:10

Computers and Technology, 29.05.2021 07:10


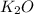 formed
formed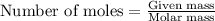
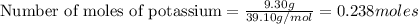
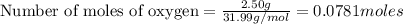
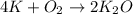
 require 1 mole of
require 1 mole of 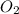
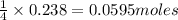 of
of 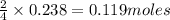 of
of