
Chemistry, 01.12.2020 20:30 Madsissabell
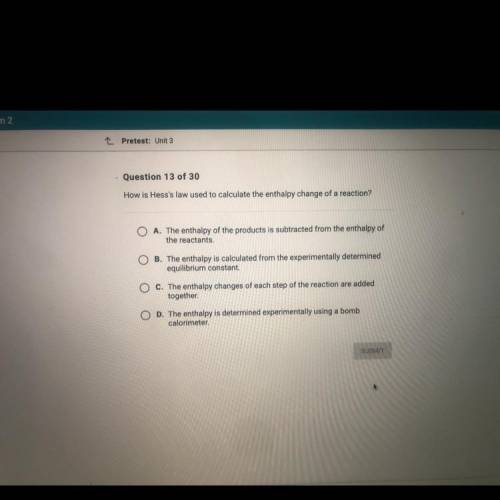
How is Hess's law used to calculate the enthalpy change of a reaction?
A. The enthalpy of the products is subtracted from the enthalpy of
the reactants,
O B. The enthalpy is calculated from the experimentally determined
equilibrium constant.
C. The enthalpy changes of each step of the reaction are added
together
O D. The enthalpy is determined experimentally using a bomb
calorimeter.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
How is Hess's law used to calculate the enthalpy change of a reaction?
A. The enthalpy of the produ...
Questions



Mathematics, 19.07.2019 18:00


Mathematics, 19.07.2019 18:00



English, 19.07.2019 18:00



Mathematics, 19.07.2019 18:00

English, 19.07.2019 18:00

Spanish, 19.07.2019 18:00

English, 19.07.2019 18:00



Health, 19.07.2019 18:00


Mathematics, 19.07.2019 18:00

Chemistry, 19.07.2019 18:00



