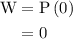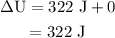Chemistry, 23.12.2019 04:31 pearlkissp1bzl8
Calculate the change in internal energy of the following system: a 100.0-g bar of gold is heated from 25 ∘c to 50 ∘c during which it absorbs 322 j of heat. assume the volume of the gold bar remains constant.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Calculate the change in internal energy of the following system: a 100.0-g bar of gold is heated fr...
Questions

English, 06.11.2020 23:20


Biology, 06.11.2020 23:20

English, 06.11.2020 23:20


Chemistry, 06.11.2020 23:20

Mathematics, 06.11.2020 23:20




Mathematics, 06.11.2020 23:20

Chemistry, 06.11.2020 23:20


Computers and Technology, 06.11.2020 23:20


Physics, 06.11.2020 23:20

Mathematics, 06.11.2020 23:20



Chemistry, 06.11.2020 23:20

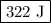
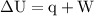 …… (1)
…… (1)
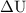 is the change in internal energy of the system.
is the change in internal energy of the system.
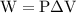 …… (2)
…… (2)
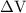 is the change in the volume of the system.
is the change in the volume of the system.