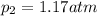Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3

Chemistry, 23.06.2019 12:30
How do you interpret a chromatogram for what mixtures contain?
Answers: 3

Chemistry, 23.06.2019 13:30
Did you mention that adding weight increased the pressure
Answers: 2
You know the right answer?
Helium gas in a cylinder is under 1.12atm pressure at 25.0C. What will be the pressure if the temper...
Questions

Biology, 20.10.2019 08:30

History, 20.10.2019 08:30



English, 20.10.2019 08:30


English, 20.10.2019 08:30


English, 20.10.2019 08:30

Mathematics, 20.10.2019 08:30


History, 20.10.2019 08:30








Chemistry, 20.10.2019 08:30