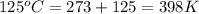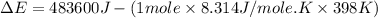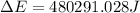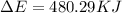Chemistry, 31.08.2019 00:00 nathaniel12
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are converted h2(g) and o2(g) against a pressure of 1 atm at 125 degrees celcius what is δe of reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are co...
Questions

Mathematics, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00


History, 29.01.2021 03:00


English, 29.01.2021 03:00

Advanced Placement (AP), 29.01.2021 03:00

Mathematics, 29.01.2021 03:00



Mathematics, 29.01.2021 03:00



Spanish, 29.01.2021 03:00

Mathematics, 29.01.2021 03:00




English, 29.01.2021 03:00

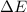 of the reaction is, 480.29 KJ.
of the reaction is, 480.29 KJ.
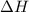 = enthalpy of the reaction = 483.6 KJ/mole = 483600 J
= enthalpy of the reaction = 483.6 KJ/mole = 483600 J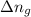 = change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole
= change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole