
Chemistry, 03.12.2020 05:20 LuluMathLover101
a metal worker uses a cutting torch that operates by reacting acetylene gas, C2H2(g), with oxygen gas, O2(g), as shown in the unbalanced equation below: C2H2(g)=O2(g)= CO2(g) = H2O(g) = heat determine the mass of 25 moles of acetylene (gram-formula mass = 26 g/mol

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2


Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
a metal worker uses a cutting torch that operates by reacting acetylene gas, C2H2(g), with oxygen ga...
Questions

Mathematics, 11.09.2020 16:01

Biology, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

World Languages, 11.09.2020 16:01

Social Studies, 11.09.2020 16:01

Biology, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Biology, 11.09.2020 16:01

English, 11.09.2020 16:01

Physics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Physics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

Mathematics, 11.09.2020 16:01

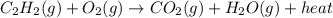
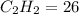 g/mol
g/mol

