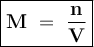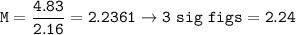Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1


You know the right answer?
Determine the molarity of a solution formed by dissolving 4.83 moles of NaCl (M=58.44 g/mol) in enou...
Questions

Physics, 20.03.2020 00:01


Mathematics, 20.03.2020 00:02

Mathematics, 20.03.2020 00:02

Mathematics, 20.03.2020 00:02

Physics, 20.03.2020 00:02




Chemistry, 20.03.2020 00:02

Mathematics, 20.03.2020 00:02

History, 20.03.2020 00:02



Mathematics, 20.03.2020 00:02

Social Studies, 20.03.2020 00:02

History, 20.03.2020 00:02

Mathematics, 20.03.2020 00:02


Computers and Technology, 20.03.2020 00:02