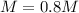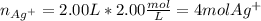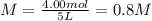Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
You have 3.00 L of a 3.12 M solution of NaCl(aq) called solution A. You also have 2.00 L of a 2.00 M...
Questions

Mathematics, 11.05.2021 17:10




Mathematics, 11.05.2021 17:10

Mathematics, 11.05.2021 17:10






Biology, 11.05.2021 17:10

English, 11.05.2021 17:10



History, 11.05.2021 17:10

History, 11.05.2021 17:10


French, 11.05.2021 17:10