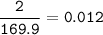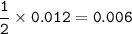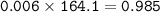Chemistry, 03.12.2020 14:00 guzmanbrandon3259
The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgNO3 → 2AgCl + Ca(NO3)2
How many grams of Ca(NO3)2 are produced from 2,000.0 grams of AgNO3?
(Molar mass of Ca = 40.1 g/mol, Cl = 35.5 g/mol, O = 16.0 g/mol, Ag = 107.9 g/mol, N = 14.0 g/mol)
(Show your calculations for full credit. Correct answers will only receive partial credit.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
The following reaction shows calcium chloride reacting with silver nitrate.
CaCl2 + 2AgNO3 → 2AgCl...
Questions


Mathematics, 06.01.2021 17:40

Mathematics, 06.01.2021 17:40


Mathematics, 06.01.2021 17:40

English, 06.01.2021 17:40



Mathematics, 06.01.2021 17:40

Mathematics, 06.01.2021 17:40


English, 06.01.2021 17:40

Social Studies, 06.01.2021 17:40

Mathematics, 06.01.2021 17:40

Mathematics, 06.01.2021 17:40




History, 06.01.2021 17:40