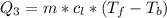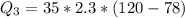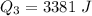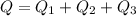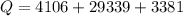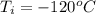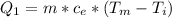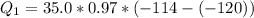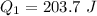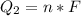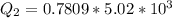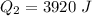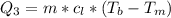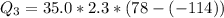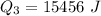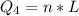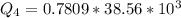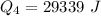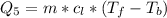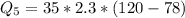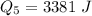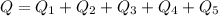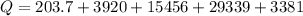Ethanol (C2H5OH) melts at –114 °C and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kJ/mol, and its enthalpy of vaporization is 38.56 kJ/mol. The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K, respectively. The average specific heat of gaseous ethanol is about 1.80 J/g-K. a. How much heat is required to convert 35.0 g of ethanol at 27 °C to the vapor phase at 120 °C? b. How much heat is required to convert the same amount of ethanol at –120 °C to the vapor phase at 120 °C?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2
You know the right answer?
Ethanol (C2H5OH) melts at –114 °C and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kJ/m...
Questions

Arts, 19.12.2020 08:20

English, 19.12.2020 08:20





Mathematics, 19.12.2020 08:20

Mathematics, 19.12.2020 08:20


Mathematics, 19.12.2020 08:20

English, 19.12.2020 08:20




Mathematics, 19.12.2020 08:20


Mathematics, 19.12.2020 08:20

English, 19.12.2020 08:20

History, 19.12.2020 08:20

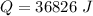
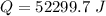
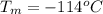
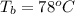
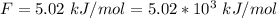
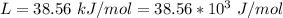
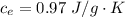
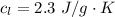
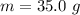
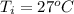
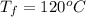
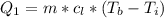
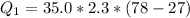
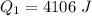
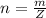
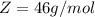
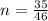
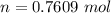
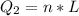
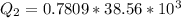
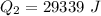
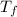 is mathematically represented as
is mathematically represented as