
Chemistry, 03.12.2020 18:40 rjennis002
The following reaction shows sodium carbonate reacting with calcium hydroxide.
Na2CO3 + Ca(OH)2 → 2NaOH + CaCO3
How many grams of NaOH are produced from 20.0 grams of Na2CO3?
(Molar mass of Na = 22.989 g/mol, C = 12.01 g/mol, O = 15.999 g/mol, Ca = 40.078 g/mol, H = 1.008 g/mol) (5 points)
a. 12.2 grams
b. 15.1 grams
c. 24.4 grams
d. 30.2 grams

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

You know the right answer?
The following reaction shows sodium carbonate reacting with calcium hydroxide.
Na2CO3 + Ca(OH)2 → 2...
Questions


English, 10.06.2020 10:57

Physics, 10.06.2020 10:57

Mathematics, 10.06.2020 10:57



Mathematics, 10.06.2020 10:57



Mathematics, 10.06.2020 10:57

Business, 10.06.2020 10:57

Computers and Technology, 10.06.2020 10:57

Arts, 10.06.2020 10:57

Mathematics, 10.06.2020 10:57


Social Studies, 10.06.2020 10:57



Mathematics, 10.06.2020 10:57

Biology, 10.06.2020 10:57

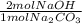 = 0.378 moles NaOH
= 0.378 moles NaOH

