
Chemistry, 03.12.2020 22:50 xezthekingx4431
An unknown metal with a mass of 54.0 g required 773 J of energy in order to change its temperature from 63.0 C to 100.0 C. What is the identity of this metal?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
An unknown metal with a mass of 54.0 g required 773 J of energy in order to change its temperature f...
Questions


Mathematics, 01.09.2019 21:30

Mathematics, 01.09.2019 21:30

Physics, 01.09.2019 21:30



Spanish, 01.09.2019 21:30






Social Studies, 01.09.2019 21:30




Mathematics, 01.09.2019 21:30

English, 01.09.2019 21:30

History, 01.09.2019 21:30

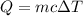
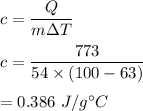
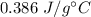 .
.



