
Chemistry, 03.12.2020 23:50 munziruddin204
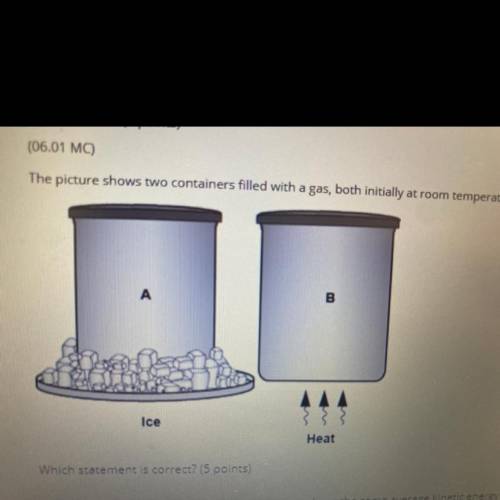
The picture shows to containers filled with a gas, both initially at room temperature
AAA
Ice
Heat
Which statement is correct? (5 points)
The gas particles in both containers have the same average kinetic energy because they have equal number of particles
The gas particles in both containers have the same average kinetic energy because they have the same volume
The average kinetic energy of the gas particles is greater in container A because it has a lower temperature
The average kinetic energy of the gas particles is greater in container 8 because its particles move faster
Od

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
The picture shows to containers filled with a gas, both initially at room temperature
AAA
Ice...
Ice...
Questions



History, 17.11.2020 23:00

Mathematics, 17.11.2020 23:00

Biology, 17.11.2020 23:00





Mathematics, 17.11.2020 23:00

Mathematics, 17.11.2020 23:00

Engineering, 17.11.2020 23:00


Health, 17.11.2020 23:00

Mathematics, 17.11.2020 23:00

History, 17.11.2020 23:00


Mathematics, 17.11.2020 23:00

Mathematics, 17.11.2020 23:00



