
Chemistry, 04.12.2020 01:00 kevonmajor
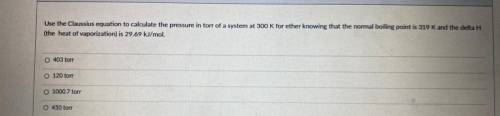
Use the Classius equation to calculate the pressure in torr of a system at 300 K for ether knowing that the normal boiling point is 319 K and the delta H
the heat of vaporization) is 29.69 kl/mol
403 ton
120 ton
1000 7 ore
450 for

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1
You know the right answer?
Use the Classius equation to calculate the pressure in torr of a system at 300 K for ether knowing t...
Questions

Health, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Advanced Placement (AP), 10.12.2020 01:00


History, 10.12.2020 01:00


Chemistry, 10.12.2020 01:00

Law, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

History, 10.12.2020 01:00



Mathematics, 10.12.2020 01:00


English, 10.12.2020 01:00



