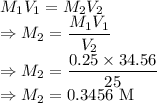Chemistry, 04.12.2020 01:00 haleyscales825
A solution of HCl with a volume of 25.00 mL is titrated to the endpoint, with 0.250 M
NaOH. If it takes 34.56 mL of NaOH, what is the original concentration of HCl in the
solution?
HCl(aq) + NaOH(aq) → H20(l)+ NaCl(aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
A solution of HCl with a volume of 25.00 mL is titrated to the endpoint, with 0.250 M
NaOH. If it t...
Questions


Social Studies, 18.12.2020 14:00


Mathematics, 18.12.2020 14:00


Mathematics, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00


History, 18.12.2020 14:00


Mathematics, 18.12.2020 14:00

Chemistry, 18.12.2020 14:00

History, 18.12.2020 14:00




Mathematics, 18.12.2020 14:00

Spanish, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

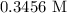
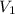 = Volume of NaOH = 34.56 mL
= Volume of NaOH = 34.56 mL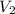 = Volume of HCl = 25 mL
= Volume of HCl = 25 mL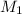 = Concentration of NaOH = 0.25 M
= Concentration of NaOH = 0.25 M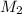 = Concentration of HCl
= Concentration of HCl