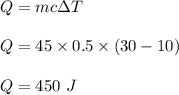Chemistry, 04.12.2020 03:40 itsmaddierae11
How much heat will a 45.00 g piece of glass require to change its temperature from 10 to 30 °C. The specific heat capacity is 0.500 J/g°C.
675 J
225 J
450 J
700 J

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
How much heat will a 45.00 g piece of glass require to change its temperature from 10 to 30 °C. The...
Questions

World Languages, 22.02.2020 14:38

English, 22.02.2020 14:40

Social Studies, 22.02.2020 14:40

Mathematics, 22.02.2020 14:40


English, 22.02.2020 14:43

History, 22.02.2020 14:43

Mathematics, 22.02.2020 14:43

Mathematics, 22.02.2020 14:43

Engineering, 22.02.2020 14:44




Geography, 22.02.2020 14:46

Mathematics, 22.02.2020 14:47


Physics, 22.02.2020 14:50

Physics, 22.02.2020 14:50

Business, 22.02.2020 14:58