
Modeling Energy Changes Student Guide on Edge
Step 2: Write and Balance the Chemical Equation.
a) Read the word equation: "Propane gas plus oxygen gas produces _."
b) Convert the word equation to a chemical equation and complete the reaction. Be sure to balance the chemical equation.
c) Record the balanced chemical equation on the Student Worksheet.
Step 3: Determine the amount of energy change in the reaction.
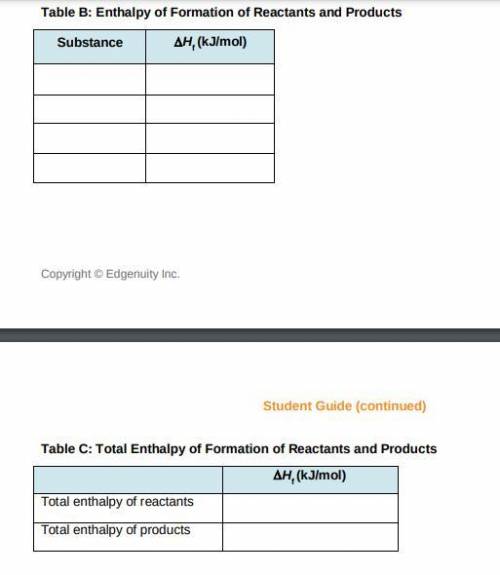
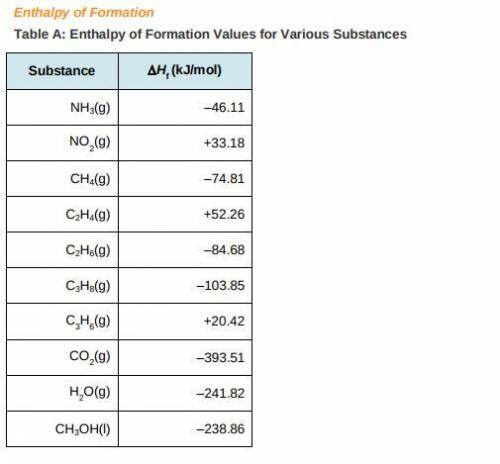
a) Use the table of enthalpy values (Table A) provided in the Student Worksheet to locate the enthalpy of formation (DeltaHt) for each reactant and each product. Record these values along with the reactants and products in Table B of the Student Worksheet.
b) Determine the total enthalpy of the reactants and the total enthalpy of the products Record these values in Table C of the Student Worksheet.
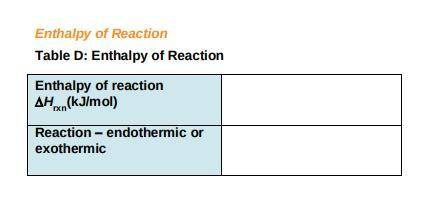
c) Use the following formula to find the net change in enthalpy for the reaction and to determine whether the reaction is endothermic is endothermic or exothermic.
DeltaHrxn= E (Delta Hf, products)- E (DeltaHf, ractants)
Record your answers in Table D.
Step 4: Model the energy change in the reaction.
a) Create an energy graph that illustrates the energy change in the reaction.
b)Construct your graph on a blank sheet of paper. Be sure to label the axes, provide a title, and identify the reactants and product on the graph.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
Modeling Energy Changes Student Guide on Edge
Step 2: Write and Balance the Chemical Equation.
Questions

Mathematics, 07.09.2021 18:20




Social Studies, 07.09.2021 18:30


English, 07.09.2021 18:30

Mathematics, 07.09.2021 18:30




Mathematics, 07.09.2021 18:30

Mathematics, 07.09.2021 18:30


Mathematics, 07.09.2021 18:30

Advanced Placement (AP), 07.09.2021 18:30

Mathematics, 07.09.2021 18:30



Mathematics, 07.09.2021 18:30



