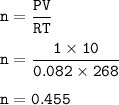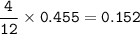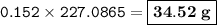Chemistry, 04.12.2020 14:00 littlemrslazy
Suppose that 10.0 L of Carbon Dioxide gas are produced by this reaction, 4C3H5N3O9 -> 12 CO2 + 10H2O + 6N2 +O2, at a temperature of -5 degrees C, and a pressure of exactly 1 atm. Calculate the mass of nitroglycerin that must have reacted in grams.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Suppose that 10.0 L of Carbon Dioxide gas are produced by this reaction, 4C3H5N3O9 -> 12 CO2 + 10...
Questions




World Languages, 30.03.2020 21:29

History, 30.03.2020 21:29

Mathematics, 30.03.2020 21:29

Mathematics, 30.03.2020 21:29

Mathematics, 30.03.2020 21:29

Mathematics, 30.03.2020 21:29







Computers and Technology, 30.03.2020 21:30