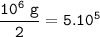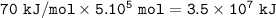Chemistry, 04.12.2020 14:00 ieyaalzhraa
In the reaction, 210 kJ of heat energy is used to form 3.0 moles of hydrogen. Calculate how much heat energy is needed to make 1000 kg of hydrogen.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1


Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
In the reaction, 210 kJ of heat energy is used to form 3.0 moles of hydrogen.
Calculate how much he...
Questions

Social Studies, 03.02.2020 03:49


English, 03.02.2020 03:49





Mathematics, 03.02.2020 03:49

Mathematics, 03.02.2020 03:49

History, 03.02.2020 03:49



Spanish, 03.02.2020 03:49


Mathematics, 03.02.2020 03:49

Mathematics, 03.02.2020 03:49


Social Studies, 03.02.2020 03:50

English, 03.02.2020 03:50

Biology, 03.02.2020 03:50