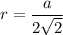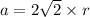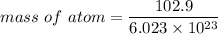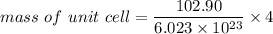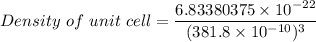Chemistry, 04.12.2020 17:00 SophomoreSareke
Rhodium crystallizes in a face-centered cubic unit cell. The radius of a rhodium atom is 135 pm. Determine the density of rhodium in g/cm3

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
Rhodium crystallizes in a face-centered cubic unit cell. The radius of a rhodium atom is 135 pm. Det...
Questions

Mathematics, 04.01.2021 17:00

English, 04.01.2021 17:00


Mathematics, 04.01.2021 17:00

Mathematics, 04.01.2021 17:00

Chemistry, 04.01.2021 17:00


English, 04.01.2021 17:00







Mathematics, 04.01.2021 17:00

Mathematics, 04.01.2021 17:00