
Chemistry, 05.12.2020 03:20 tgraveslaylay2743
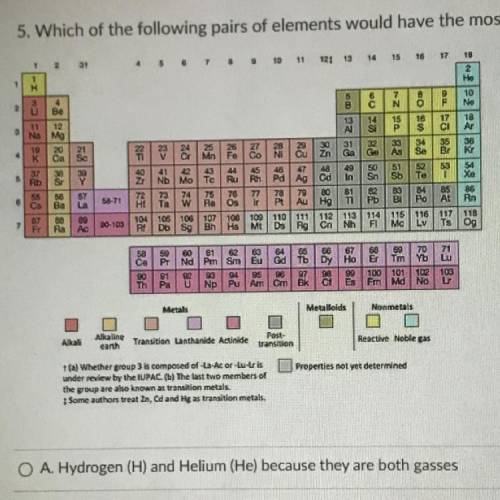
Which of the following pairs of elements would have the most similar properties?
A. Hydrogen (H) and Helium (He) because they are both gasses
B. Sodium (Na) and Potassium (K) because they have similar reactivity
C. Antimony (Sb) and Silicon (Si) because they are both metalloids
D. Aluminum (Al) and Magnesium (Mg) because they are both silver

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
Which of the following pairs of elements would have the most similar properties?
A. Hydrogen (H) an...
Questions




History, 31.01.2020 17:00

Biology, 31.01.2020 17:00





Mathematics, 31.01.2020 17:00


Biology, 31.01.2020 17:01

Social Studies, 31.01.2020 17:01






History, 31.01.2020 17:01




