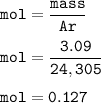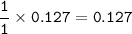Chemistry, 05.12.2020 03:40 willcoop6470
In the lab, a student collects hydrogen gas over water in a eudiometer. The hydrogen gas is produced when a piece of magnesium metal reacts with excess hydrochloric acid. Part 1: (a) Write the balanced chemical equation for this reaction. Include the states of matter. Mg(s) + 2HCl (aq) — H2 (8) +MgCl, (aq) Part 2 out of 2 (b) How many moles of hydrogen gas are collected if 3.09 g of magnesium metal is used in the reaction? Report prc Moles of hydrogen gas mol H2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
In the lab, a student collects hydrogen gas over water in a eudiometer. The hydrogen gas is produced...
Questions


Computers and Technology, 18.11.2020 20:00

English, 18.11.2020 20:00


Mathematics, 18.11.2020 20:00





Mathematics, 18.11.2020 20:00

Mathematics, 18.11.2020 20:00

Biology, 18.11.2020 20:00




Biology, 18.11.2020 20:00

History, 18.11.2020 20:00



Chemistry, 18.11.2020 20:00