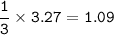Chemistry, 05.12.2020 03:50 nell1234565
How many moles of nitrogen gas would be produced if 3.27 moles of copper(II) oxide were reacted with excess ammonia in the following chemical reaction? 2 NH3(g) + 3 CuO (s) – 3 Cu(s) + N2(g) + 3 H2O(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 21:30
Karen has discovered a new organism in the amazon rainforest. it moves by itself, is a consumer, and its cells do not have a cell wall. in what kingdom would this newly discovered organism belong? a) amazon b) animalia c) fungi d) plantae
Answers: 1
You know the right answer?
How many moles of nitrogen gas would be produced if 3.27 moles of copper(II) oxide were reacted with...
Questions

Mathematics, 14.12.2020 21:10


Chemistry, 14.12.2020 21:10

Biology, 14.12.2020 21:10


Mathematics, 14.12.2020 21:10


Computers and Technology, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10


Mathematics, 14.12.2020 21:10

History, 14.12.2020 21:10


Spanish, 14.12.2020 21:10

English, 14.12.2020 21:10