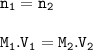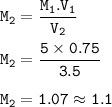Chemistry, 05.12.2020 04:10 huneymarie
A student has 0.75 L of a 5.0 M solution and dilutes it to make 3.5 liters. What’s the molarity of the diluted solution?
1.1 M
2.0 M
1.6 M
.93 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
A student has 0.75 L of a 5.0 M solution and dilutes it to make 3.5 liters. What’s the molarity of t...
Questions


Mathematics, 28.01.2021 17:20



Computers and Technology, 28.01.2021 17:20

Mathematics, 28.01.2021 17:20







Mathematics, 28.01.2021 17:20

Mathematics, 28.01.2021 17:20


Mathematics, 28.01.2021 17:20

Mathematics, 28.01.2021 17:20

Mathematics, 28.01.2021 17:20


Social Studies, 28.01.2021 17:20