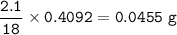Chemistry, 05.12.2020 14:00 ohartshorn3670
Vitamin C contains only carbon, hydrogen, and oxygen. When a 1.00 g was combusted, 1.4991 g of CO2 and 0.4092 g of H2O were obtained. Determine the empirical formula for this vitamin.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1


Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
Vitamin C contains only carbon, hydrogen, and oxygen. When a 1.00 g was combusted, 1.4991 g of CO2 a...
Questions



Mathematics, 16.03.2020 15:26

Mathematics, 16.03.2020 15:26

Geography, 16.03.2020 15:26

Mathematics, 16.03.2020 15:27

Mathematics, 16.03.2020 15:27

English, 16.03.2020 15:28

Advanced Placement (AP), 16.03.2020 15:28


Mathematics, 16.03.2020 15:30


Mathematics, 16.03.2020 15:31

Social Studies, 16.03.2020 15:31

Mathematics, 16.03.2020 15:32


Mathematics, 16.03.2020 15:33

Arts, 16.03.2020 15:34