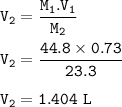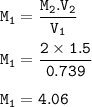Chemistry, 05.12.2020 14:00 najeezubair0666
Part 1: What is the final volume in milliliters when 0.730 L of a 44.8 % (m/v) solution is diluted to 23.3 % (m/v)? part 2:A 739 mL NaCl solution is diluted to a volume of 1.50 L and a concentration of 2.00 M . What was the initial concentration?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Part 1: What is the final volume in milliliters when 0.730 L of a 44.8 % (m/v) solution is diluted t...
Questions


Mathematics, 09.01.2020 01:31

History, 09.01.2020 01:31


Mathematics, 09.01.2020 01:31

Biology, 09.01.2020 01:31

Biology, 09.01.2020 01:31

History, 09.01.2020 01:31

English, 09.01.2020 01:31

Mathematics, 09.01.2020 01:31


Mathematics, 09.01.2020 01:31

History, 09.01.2020 01:31

Health, 09.01.2020 01:31


Computers and Technology, 09.01.2020 01:31


Computers and Technology, 09.01.2020 01:31