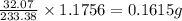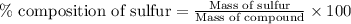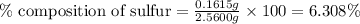Chemistry, 05.11.2019 02:31 FlyingPig14
A2.5600 gram sample of a sulfur-containing compound is analyzed by precipitating the sulfur as baso4. if 1.1756 g of baso4 are obtained, what is the percentage of sulfur in the sample?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
A2.5600 gram sample of a sulfur-containing compound is analyzed by precipitating the sulfur as baso4...
Questions


English, 01.02.2022 14:00

Computers and Technology, 01.02.2022 14:00

Mathematics, 01.02.2022 14:00


Biology, 01.02.2022 14:00

Mathematics, 01.02.2022 14:00



Mathematics, 01.02.2022 14:00

Mathematics, 01.02.2022 14:00

English, 01.02.2022 14:00


English, 01.02.2022 14:00



Mathematics, 01.02.2022 14:00