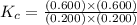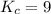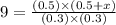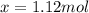Chemistry, 09.12.2019 05:31 wednesdayA
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas) and 0.200 mol of each of the reactants (carbon monoxide and water vapor) in a 1.00-l container. this is the equation: co(g)+h2o(> < -- co2(g) + h2(g). how many moles of carbon dioxide would have to be added at constant temperature and volume to increase the amount of carbon monoxide to 0.300 mol?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
An equilibrium mixture contains 0.600 mol of each of the products (carbon dioxide and hydrogen gas)...
Questions




English, 12.02.2021 21:10

Biology, 12.02.2021 21:10





History, 12.02.2021 21:10





Mathematics, 12.02.2021 21:10





Social Studies, 12.02.2021 21:10

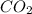 added will be 1.12 mole.
added will be 1.12 mole.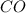 and
and 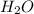 at equilibrium = 0.200 mol
at equilibrium = 0.200 mol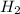 at equilibrium = 0.600 mol
at equilibrium = 0.600 mol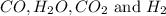 at equilibrium.
at equilibrium.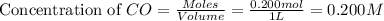
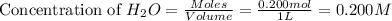
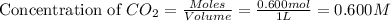
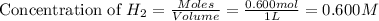
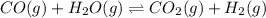
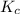 will be,
will be,![K_c=\frac{[H_2][CO_2]}{[CO][H_2O]}](/tpl/images/0409/6473/e1151.png)