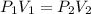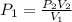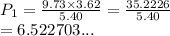A sample of argon, Ar, has a volume of 5.40 L with an unknown pressure. The gas has a volume of 9.73 L when the pressure is 3.62 atm,
with no change in temperature and amount of gas. What was the initial pressue in atm of the gas?
O a. 6.523 atm
O b. 6.52 atm
Oc 2.009 atm
Od. 2,01 atm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

You know the right answer?
A sample of argon, Ar, has a volume of 5.40 L with an unknown pressure. The gas has a volume of 9.73...
Questions

Social Studies, 27.05.2021 05:20

Mathematics, 27.05.2021 05:20





Mathematics, 27.05.2021 05:20

Health, 27.05.2021 05:20





Mathematics, 27.05.2021 05:20

Mathematics, 27.05.2021 05:20

Mathematics, 27.05.2021 05:20

Spanish, 27.05.2021 05:20

Mathematics, 27.05.2021 05:20

English, 27.05.2021 05:20

Mathematics, 27.05.2021 05:20

Mathematics, 27.05.2021 05:20