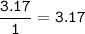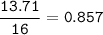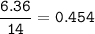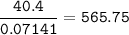Chemistry, 06.12.2020 05:00 timithythaxton
A 40.4 g sample of a protein contains 17.16 g of carbon, 3.17 g of hydrogen, 13.71 g of oxygen, and the rest being nitrogen. This 40.4-gram sample is known to be 0.07141 moles. Determine the molecular formula of this protein.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3
You know the right answer?
A 40.4 g sample of a protein contains 17.16 g of carbon, 3.17 g of hydrogen, 13.71 g of oxygen, and...
Questions



Biology, 30.01.2021 17:10

Chemistry, 30.01.2021 17:10

Chemistry, 30.01.2021 17:10

Mathematics, 30.01.2021 17:10



Mathematics, 30.01.2021 17:10

Mathematics, 30.01.2021 17:10

Mathematics, 30.01.2021 17:10

Mathematics, 30.01.2021 17:10

Physics, 30.01.2021 17:10





Mathematics, 30.01.2021 17:20


Mathematics, 30.01.2021 17:20