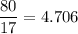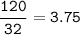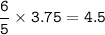Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
In a lab experiment 80.0 g of ammonia [NH3] and 120 g of oxygen are placed in a reaction vessel. At...
Questions

Mathematics, 04.02.2020 06:04

English, 04.02.2020 06:04





Biology, 04.02.2020 06:04


Social Studies, 04.02.2020 06:04


History, 04.02.2020 06:04

Mathematics, 04.02.2020 06:04

Mathematics, 04.02.2020 06:04

Geography, 04.02.2020 06:04

English, 04.02.2020 06:04