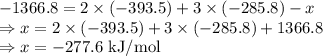Chemistry, 06.12.2020 05:40 sebastianapolo5
Ethanol undergoes combustion in oxygen to produce carbon dioxide gas and liquid water. The standard heat of combustion of ethanol, c2h5oh(l), is -1366.8 JK/mol. Given that [co2(g)] = -393.5kg/mol and [h20(l) = -285.8 KJ/ mol, what is the standard enthalpy of formation of ethanol?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
Ethanol undergoes combustion in oxygen to produce carbon dioxide gas and liquid water. The standard...
Questions

History, 02.02.2021 21:00


Mathematics, 02.02.2021 21:00


Mathematics, 02.02.2021 21:00

Mathematics, 02.02.2021 21:00


Biology, 02.02.2021 21:00

English, 02.02.2021 21:00

History, 02.02.2021 21:00

Mathematics, 02.02.2021 21:00


Mathematics, 02.02.2021 21:00

Spanish, 02.02.2021 21:00


English, 02.02.2021 21:00

Mathematics, 02.02.2021 21:00

Biology, 02.02.2021 21:00

Arts, 02.02.2021 21:00

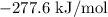
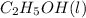 = -1366.8 kJ/mol
= -1366.8 kJ/mol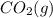 = -393.5 kJ/mol
= -393.5 kJ/mol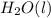 = -285.8 kJ/mol
= -285.8 kJ/mol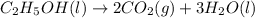
 is the standard enthalpy of formation of ethanol
is the standard enthalpy of formation of ethanol