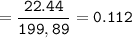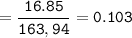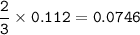Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
A mixture of 22.44 g of CaBr2 and 16.85 g Na3PO4 is used in the following reaction. Determine the ma...
Questions

Mathematics, 11.03.2021 05:10

History, 11.03.2021 05:10

English, 11.03.2021 05:10

Social Studies, 11.03.2021 05:10

English, 11.03.2021 05:10


Chemistry, 11.03.2021 05:10


Mathematics, 11.03.2021 05:10

Mathematics, 11.03.2021 05:10


Mathematics, 11.03.2021 05:10



Mathematics, 11.03.2021 05:10


Mathematics, 11.03.2021 05:10


Mathematics, 11.03.2021 05:10

Mathematics, 11.03.2021 05:10