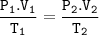Chemistry, 06.12.2020 15:50 collegebound3506
A balloon originally had a volume of 4.39 L at 44C and a pressure of 729 torr  to what temperature must the balloon be cooled to reduce its volume to 3.78 L of the new pressure is at 1.0 atm

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
A balloon originally had a volume of 4.39 L at 44C and a pressure of 729 torr  to what temperatur...
Questions

Social Studies, 28.08.2019 20:30



History, 28.08.2019 20:30

Mathematics, 28.08.2019 20:30

Physics, 28.08.2019 20:30

History, 28.08.2019 20:30

History, 28.08.2019 20:30




English, 28.08.2019 20:30

History, 28.08.2019 20:30

English, 28.08.2019 20:30



History, 28.08.2019 20:30

Chemistry, 28.08.2019 20:30