
Chemistry, 06.12.2020 16:10 RockieLuv8707
Calculate the actual mass of one atom of carbon if 12g of cardron contains one mole of carbon.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Calculate the actual mass of one atom of carbon if 12g of cardron contains one mole of carbon....
Questions

Mathematics, 26.02.2021 07:50

Health, 26.02.2021 07:50

Advanced Placement (AP), 26.02.2021 07:50

Chemistry, 26.02.2021 07:50

Mathematics, 26.02.2021 07:50

History, 26.02.2021 07:50

Mathematics, 26.02.2021 07:50

Mathematics, 26.02.2021 07:50


Mathematics, 26.02.2021 07:50


Mathematics, 26.02.2021 07:50

Physics, 26.02.2021 07:50


Mathematics, 26.02.2021 08:00


Mathematics, 26.02.2021 08:00

History, 26.02.2021 08:00

Mathematics, 26.02.2021 08:00

Mathematics, 26.02.2021 08:00

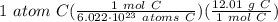 = 1.99435 × 10⁻²³ g C
= 1.99435 × 10⁻²³ g C

