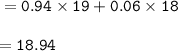Chemistry, 06.12.2020 16:50 davienwatson8
A sample of fluorine contains isotopes with different masses; F-19 and F-18. In the sample 94% of the atoms are F-19. Calculate the relative mass of the sample.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1

You know the right answer?
A sample of fluorine contains isotopes with different masses; F-19 and F-18. In the sample 94% of th...
Questions

Mathematics, 10.10.2019 19:30




Mathematics, 10.10.2019 19:30


History, 10.10.2019 19:30


English, 10.10.2019 19:30



History, 10.10.2019 19:30




English, 10.10.2019 19:30

Mathematics, 10.10.2019 19:30

Mathematics, 10.10.2019 19:30

Social Studies, 10.10.2019 19:30